In the following, packaging materials are understood to be materials which are used for the packaging of industrially produced finished medicinal products. These packaging materials must fulfil specific requirements both individually and in combination with each other.
The most important requirement is to safeguard the tested and approved product until it is used by the user. The product specification must remain guaranteed within its defined limits until the expiry date!
In addition, the packaging materials must at all times comply with the specification (approval) reported to and deposited with the authorities. Deviations from this must be reported to the authorities and in serious cases lead to a recall from the market concerned.
Requirements, which mainly relate to material quality and functionality, result from the processing of the packaging materials on high-performance packaging machines, the use along the distribution chain from the manufacturer via the dispatch warehouse to the wholesaler and on to the pharmacy. Of course, easy and safe handling by the patient must also be guaranteed.
Further requirements concerning aspects of user-friendliness and drug safety, such as Braille, child safety, fraud control, counterfeit protection and tamper-evident packaging have been added in recent years.
Primary packaging is understood to be all packaging materials that come into contact with the product. These include plastic and aluminium films, glass of various designs and qualities, plastic and elastomer closures, tubes made of aluminium, plastics and composite materials, containers made of plastic, aluminium and sheet metal and their closures.
All other packaging materials of a finished medicinal product, such as package inserts, brochures, labels, adhesive labels, folding boxes and add-on parts (e.g. dosage aids, applicators...) are referred to as secondary packaging materials.
All printed packaging materials are of particular importance, since these packaging materials contain product-specific information and errors can have far-reaching consequences. According to the definitions in the EU GMP guidelines, packaging materials are not considered to be starting materials. Nevertheless, the same requirements should apply to the handling of packaging material as to starting materials. For example, Chapter 5.45 of the EU GMP Guide (March 2015) states, "As much attention should be paid to the purchase, handling and control of primary and printed packaging material as to the starting materials." This is understandable, as some of the packaging materials are directly related to the product and labeling errors or misclassifications can cause significant problems for the user. The responsibilities for handling packaging materials are clearly regulated.
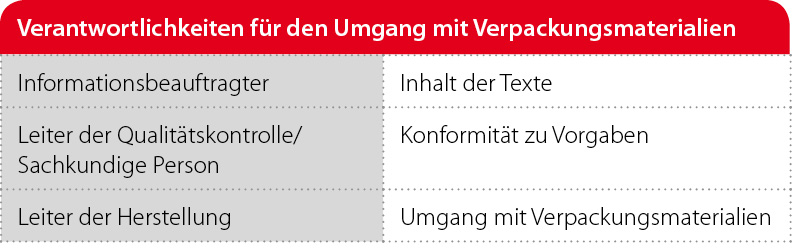
With regard to the packaging materials and their definition, there are in part very different forms of organisation depending on the company. Depending on the size of the company and the respective orientation, the tasks can be evaluated differently and thus also organized differently. In the case of research-based pharmaceutical manufacturers with a high volume of development work, people with specialist knowledge will already be looking after the primary packaging materials in pharmaceutical development and usually also, due to their technological affinity, the disposable medical products. If there is no permanent need for this specialist knowledge, "purchasing" this knowledge from other areas of the company (production) or from outside the company is a conceivable option.
Tasks and function
The primary packaging material protects the packaged product from environmental influences and secures the product according to its specification until it is used by the user.
Since the primary packaging materials are in contact with the product, there must be no interaction between the product and the material of the packaging materials. The principle possible interactions adsorption, absorption, diffusion and migration are shown schematically in the figure.
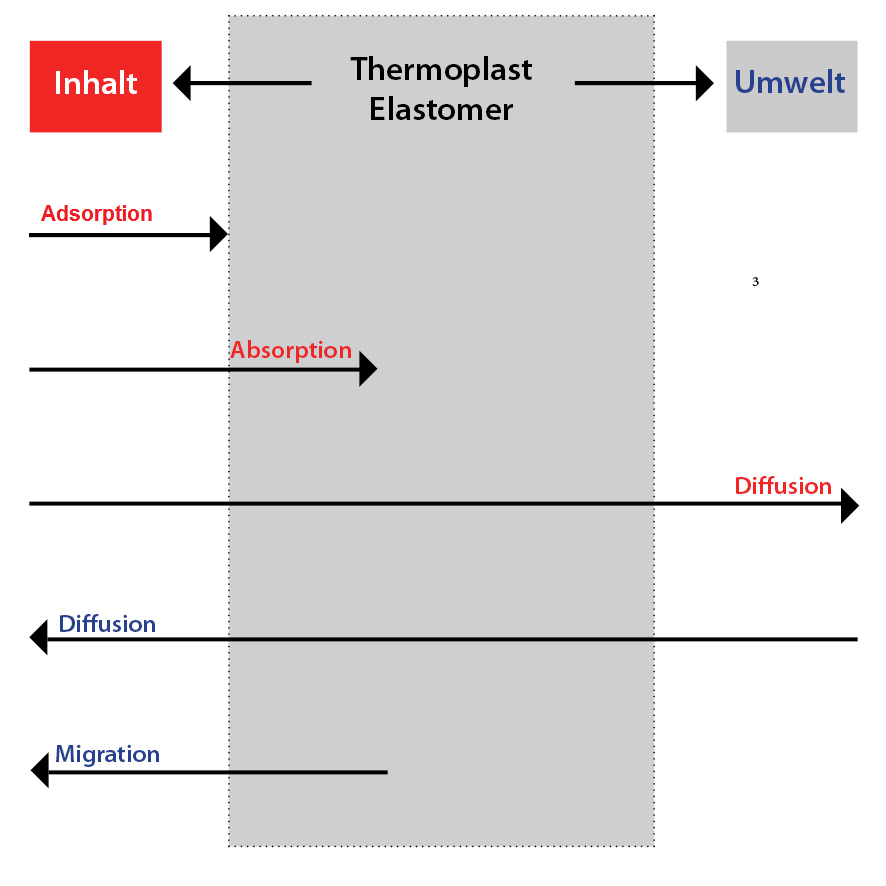
Adsorption and absorption can affect active ingredients, preservatives, excipients and solvents. This can result in loss of active ingredients, reduction of antimicrobial properties, decomposition due to loss of stabilizing components and loss of aroma on the drug. Consequential effects on the packaging material may include swelling, changes in the mechanical properties, stress corrosion, discoloration and a change in permeability.
Diffusion processes usually involve solvents diffusing out of the drug and hydrogen, oxygen and carbon dioxide diffusing into the drug from outside. This can lead to oxidative degradation of the active ingredients in the drug, a change in the pH value, decomposition by hydrolysis, absorption of foreign aromas and a change in odour or taste components. The appearance of the product may also change.
Another undesirable interaction is migration. This is the migration of low molecular weight substances (e.g. plasticizers) to the surface of plastics (in this case the packaging material) or into surrounding media (in this case the drug). In most cases, additives (such as stabilizers, lubricants, plasticizers, dyes, crosslinkers, vulcanization accelerators, fillers, catalysts, antistatic agents, UV adsorbers) migrate from the packaging material into the drug. As a consequence of migration, discoloration may occur on the drug due to the colorlessness of the packaging material, turbidity and precipitation, decomposition of the active ingredient as well as changes in odor and taste. On the packaging material, migration may cause colour changes due to pigment loss, embrittlement due to plasticizer release, ageing due to loss of stability and changes in permeability.
In order to be able to correctly evaluate interactions between medicinal products and packaging materials, extensive knowledge of both the ingredients of the medicinal product and the packaging materials is required.
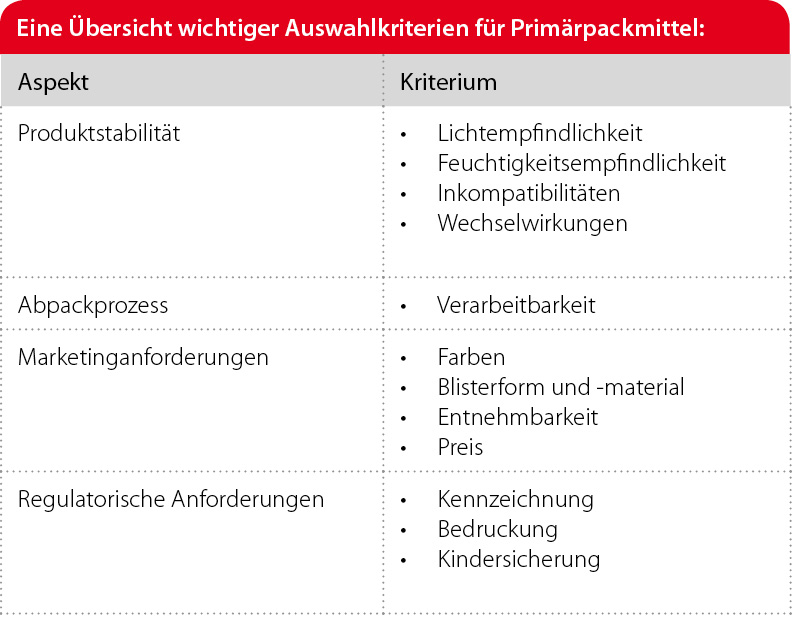
The selection of primary packaging materials begins early on in the pharmaceutical development process. The responsible product developer will already identify specific product requirements from the product composition and the first orientational shelf life tests and then select the primary packaging materials. For example, the drugs may be sensitive to light or moisture or have known incompatibilities. Nevertheless, it is only after storage tests over a longer period of time, which mimic the climatic conditions of global climates, that changes in the product and/or the primary packaging become apparent.
The risk of a change in the primary packaging, due to insufficient storage results, can be minimized by selecting and using different primary packaging material designs during the early phase of pharmaceutical development. Later changes to the primary packaging are usually associated with great expense and, in extreme cases, hinder the early offering of a new drug or the further marketing of a drug that has already been offered.
It also makes sense to take into account the legal requirements of the target markets, specific requirements for the function of the product (e.g. suitable for children, suitable for senior citizens) and existing production possibilities at an early stage in the selection process for the primary packaging materials.
An extremely comprehensive set of standards has existed for decades in the field of primary packaging materials for parenteral applications and their accessories. Based on German (DIN) standards, a packaging standard that can be used at any time has been described at European (EN) and, even more importantly, at international (ISO) level. The standards contain specifications on:
-
Design, dimensions, tolerances and functionality,
-
identification of components in elastomers,
-
physical, chemical and biological requirements,
-
quality management systems.
For day-to-day work, we recommend the DIN pocketbook 231 "Primary packaging materials and accessories for pharmaceutical applications" from Beuth Verlag and researching the current standards.
There, all essential primary packaging materials for this field of application, such as ampoules, tubular vials, injection and infusion bottles made of moulded glass as well as the corresponding rubber stoppers and crimp caps, prefilled syringes and so-called pens are described and specified in detail.
Beyond these standards, special solutions may be required for special requirements. Here it is always advisable to work together with the leading companies in this market segment and to jointly pursue further developments.
Liquid pharmaceutical products such as juices, drops or emulsions for oral or external use are filled in glass containers. For glass bottles, there are standardized containers and neck finishes as well as likewise standardized closures made of metal and plastic (see DIN pocketbook 231). Product-related droppers, pipette assemblies, spray attachments and dosing systems (e.g. siphons) are available in a wide variety of designs or are newly developed for specific products.
Special importance is always attached to the interaction between the glass mouth and the sealing element of the closure. Ideally, the closure design seals at different points of the neck finish and thus prevents unintentional leakage of the product during storage and use.
Tasks and function
Secondary packaging is the term used to describe all the components of a prepackage that do not come into direct contact with the product. Essentially, these are labels, directions for use (package insert) and folding cartons.
Their main purpose is to protect the primary packaging, to label the medicinal product and to ensure the cost-effective production of a medicinal product package as well as effective handling along the distribution chain to the consumer.
Labelling is regulated by the regulatory authorities and is largely a component of the marketing authorisation documents.
A significant savings effect in both the production and processing of the packaging material is achieved through the common use of standards.
Material, dimensions, construction and presentation/colours can be standardised.
In the case of glass bottles and the associated closures, standardisation has resulted in a material and design standard which is used by many customers. For all other forms of packaging, especially blister packs, a company and/or site standard is usually used, thereby neglecting a large savings potential.
All packaging materials can make a very valuable contribution to protecting against counterfeit medicines. Since the originality of the medicinal product (tablet, juice, ointment...) cannot be detected without sometimes very extensive testing, the packaging material must take on this task.
A distinction must be made here between overt and covert anti-counterfeiting features. Furthermore, it is important to what extent these features are known. Ideally, the patient should be able to recognize the original packaging as such.
An "integrated" protection against counterfeiting is achieved by consistent design of all packaging materials and knowledge of special features of the packaging material production and the own packaging process as well as the technology used. Since retention samples of each packaged batch must be stored, it makes sense to store at least one retention sample for checking counterfeits and to use it if necessary. A trained specialist will be able to detect deviations from the original very quickly.
For control by laypersons, the adoption of technologies from the high-security sector (money production, ID and document security) and their adaptation into the manufacturing practice of packaging materials for medicinal products is recommended, where permitted. It should be noted that although these technologies raise the hurdles for counterfeiters, they can only be effective as part of an overarching anti-counterfeiting concept.
Both the selection of the technologies and their implementation in the packaging materials is a special task and is subject to secrecy.
The coming years will show to what extent the incipient serialization and control of pharmaceutical packaging in some countries will change the requirements for counterfeit protection.
A correctly prepared packaging specification represents a comprehensive description of the packaging and serves as a specification for the respective packaging suppliers. Furthermore, it is the basis for the quality inspections at the packaging supplier and for the incoming goods inspection at the pharmaceutical manufacturer.
The correct assignment of all packaging material specifications and imprints (lot number, expiry date, price) in a packaging parts list is of particular importance as a specification for the packaging company.
As a result, it is necessary to entrust well-trained persons with the coordination and summary of these specifications and documents. For packaging material assortments with 1,000 and more individual components, this requires an IT-supported procedure.
Since the essential requirements for the packaging materials arise from the packaging process and the packaging equipment used for this purpose, the persons responsible for the packaging material specification will usually be found close to production. The packaging material specifications and packaging regulations required for packaging must be available in authorized written form, since they are also required for release by the person responsible for quality. The specifications for printing the respective packaging materials must be integrated into these mostly technical specifications. Today, this should always be a pdf template created by typesetting programs. Furthermore, specifications from the packaging manufacturer and the logistics/purchasing departments are necessary for a sufficient packaging specification.
Depending on the range of medicinal products to be packaged, a change frequency of three to four changes per year must be taken into account, especially for printed packaging materials such as leaflets and folding boxes. The creation of packaging material specifications requires a clear and robust process that can easily cope with an extreme volume of changes at short notice.
In principle, contents of the packaging material specification of primary packaging materials are already specified in the pharmaceutical technology documents and, as a result, in the registration documents of the medicinal product. Important aspects for the selection of the primary packaging material have been described in the chapter Primary Packaging Material. Depending on the respective duration of use of the drug, the number of products to be packed and thus the equipment of the packaging is determined.
A suitable packaging system is then selected within the framework of these specifications. The standards (such as packaging dimensions) of this machine are also required for the packaging specifications.
In very many cases, it is also possible to access data sheets from the packaging material manufacturers and/or packaging material manufacturers (e.g. paper and cardboard specifications, adhesive descriptions for adhesive labels). The parameters that are important for the packaging process can be taken from these or a cross-reference can be made to the data sheets with a clear assignment, e.g. via the date of issue.
Furthermore, in many areas there are industrial standards (DIN, EN and ISO standards), which form a very good basis for packaging specifications.
In the meantime, country-specific specifications (font size, text size, colours, ...) have a considerable influence on the dimensions and presentation of the printed packaging materials (blisters, labels, package inserts and folding boxes). In some cases, this even means that other packaging techniques have to be used (e.g. a plano package insert becomes a multiple-folded in/out insert). This in turn has an impact on the necessary packaging technology.
In most cases, further specifications from the area of logistics (such as delivery times) and purchasing (conditions) are also mapped in the packaging material specifications.
To ensure the uniqueness of a packaging material, multi-digit packaging material numbers are assigned, which should change with each change process. The mixing of similar packaging materials is prevented by means of visual marks and bar codes or 2D matrix codes and their control both at the packaging material manufacturer and during the packaging process.
Some examples of packaging material characteristics that need to be defined in the specification are shown in Figure 4. The inspection points are usually checked during the packaging inspection at the packaging manufacturer and documented by means of a certificate. Certain test points may also be checked during the incoming inspection of the pharmaceutical manufacturer.

The most important requirement for the packaging of a medicinal product is the protection of the product so that compliance with the initial quality in accordance with the specifications can be ensured throughout the entire shelf life. Another essential aspect is clear labelling in terms of patient safety. In addition, there are requirements for user-friendliness and drug safety, such as child-proofing or tamper-evident closure.
When selecting the primary packaging material, particular attention must be paid to possible interactions with the drug. Data on this are usually available from the early stages of drug development.
Secondary packaging materials serve to protect the primary packaging and to label the medicinal product. The contents of the labelling are regulated by the regulatory authorities and are for the most part part part of the marketing authorisation documents.
The great variety of shapes, materials and dimensions of packaging materials requires a high degree of technical processing. By defining and using uniform standards, great savings potentials could be realized here.
On the other hand, the prevention of counterfeit medicinal products requires individual and sometimes complex measures to ensure the originality of the medicinal product and to make it recognisable to the consumer.
The packaging specification represents a comprehensive description of the packaging and serves as a specification for the respective packaging suppliers. Furthermore, it is the basis for the quality inspections at the packaging supplier and during the incoming inspection at the pharmaceutical manufacturer.
Due to the large number of special methods, testing the quality of primary packaging materials in accordance with specifications is a great challenge for the pharmaceutical manufacturer and is therefore usually carried out at the packaging material manufacturer's premises. This requires an appropriate supplier qualification and contract design. At the pharmaceutical manufacturer, a reduced inspection is then carried out as part of the incoming inspection.
The handling of packaging materials is associated with high risks due to the danger of sub-mixing and confusion. These risks can be reduced by dedicated procedures in the areas of storage, labelling and transport and by professionally qualified personnel.
Source:ppt-kleissendorf
Learn more?
Would you like to learn more about our product range or do you have any questions? Do you need an individual offer or information about ordering, delivery and payment conditions? Contact us by phone, email or live chat.
