EVERIC® optimized vials
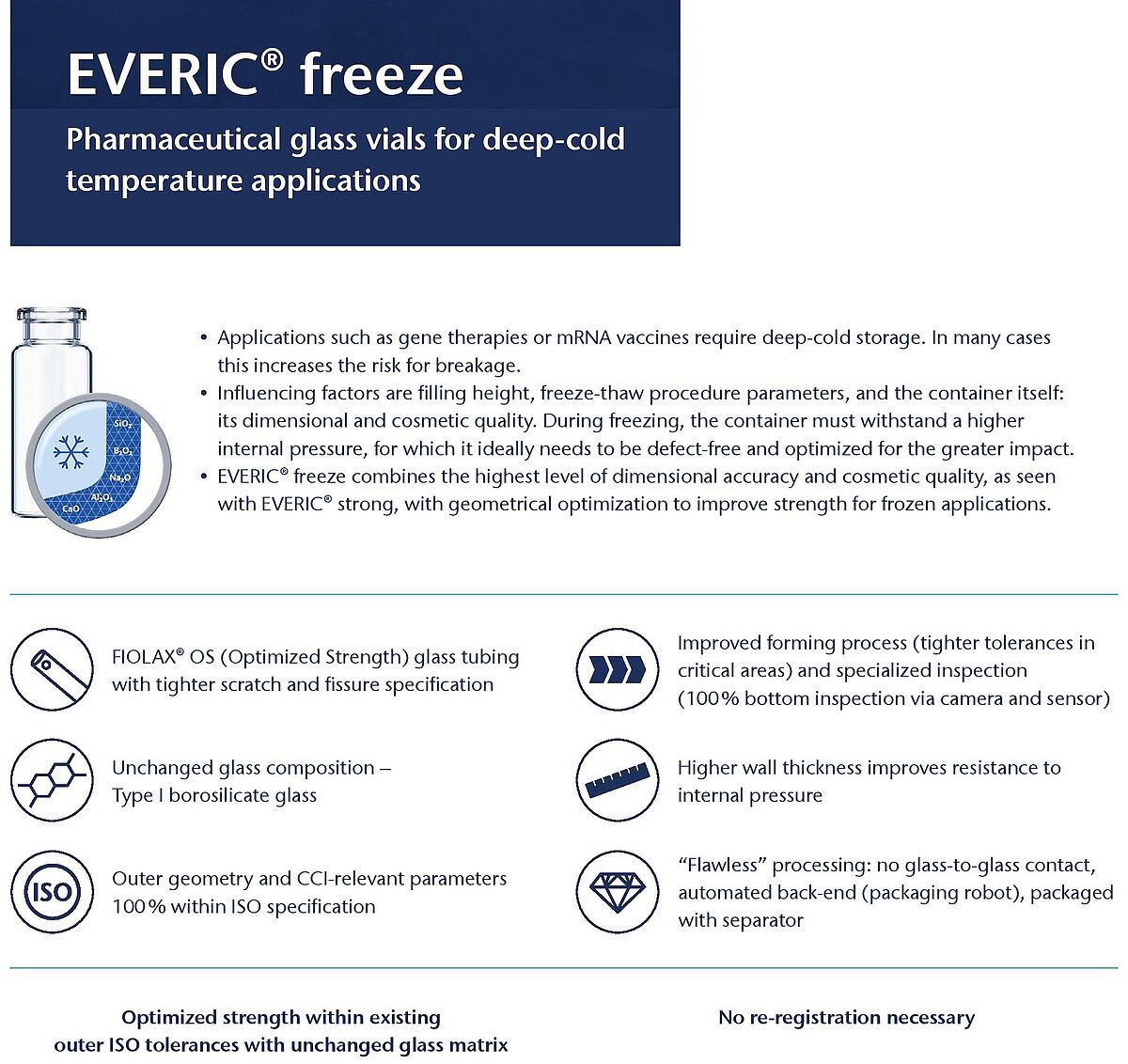
SCHOTT EVERIC® pure | care | freeze | strong & smooth are ideal injection vials for all types of drugs. Proven drug stability, especially for sensitive drugs and drugs with low filling volumes. SCHOTT EVERIC® vials ensure drug stability by using FIOLAX® CHR (Controlled Hydrolytic Resistance for EVERIC® care and pure) or FIOLAX® OS (Optimized Strength for EVERIC® freeze and strong & smooth) glass tubing, which has a higher chemical stability than standard borosilicate glass without changing the composition.
SCHOTT EVERIC® vials are produced on a special delamination-controlled production line using SCHOTT's patented technology, which provides tighter control during the process. This creates a homogeneous wall, even in the heel zone (the area of a vial close to the bottom). For validation and product release, SCHOTT has also developed and patented the Quicktest, which quantifies the risk of leachability / delamination of each production batch.
SCHOTT EVERIC® vials are also available in sterile and ready-to-use SCHOTT adaptiQ® RTU version.


Learn more?
Would you like to learn more about SCHOTT AG products or do you have any questions? Do you need an individual offer or information about ordering, delivery and payment conditions? Contact us by phone, email or live chat.