TopLyo® Vials
Freeze-drying is an essential process in the biopharmaceutical industry that accelerates a drug's time to market and increases shelf-life stability. However, lyophilization can cause fogging of the glass container, which can lead to problems during the crucial testing phase of drug manufacturing.
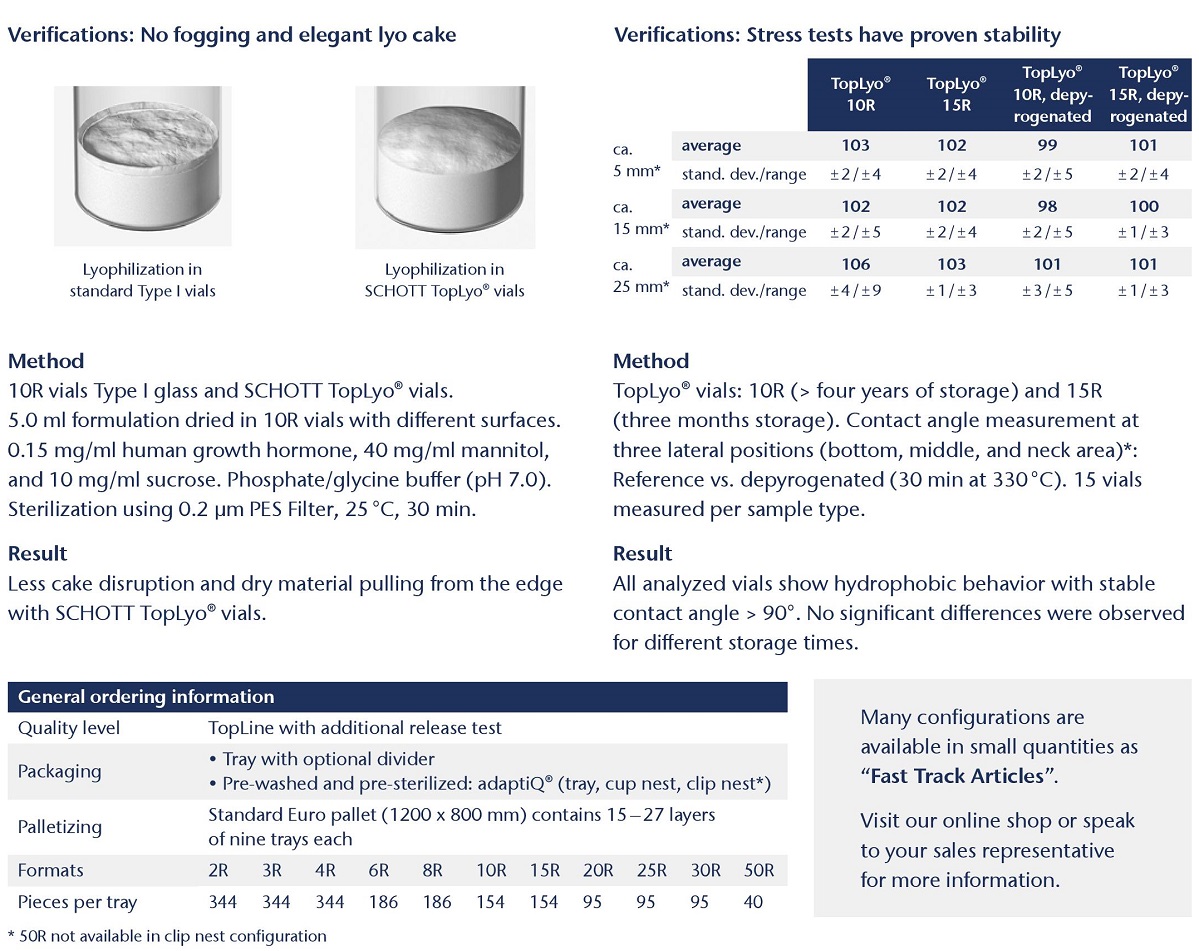
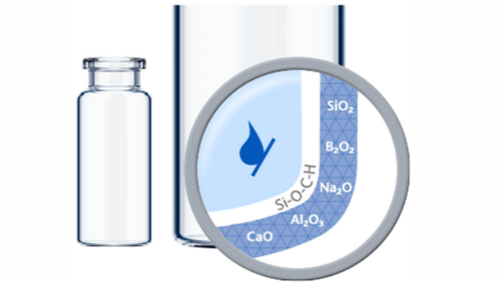
SCHOTT TopLyo® vials have a chemically uniform hydrophobic coating that results in less fogging during the lyophilization process and less destruction of the lyo cake. This improves the efficiency of automated inspection and leads to increased patient safety. In addition, TopLyo® offers reduced residual drug volumes and higher dosing accuracy after reconstitution.
SCHOTT TopLyo® Vials are also available in sterile and ready-to-use SCHOTT adaptiQ RTU version.
-
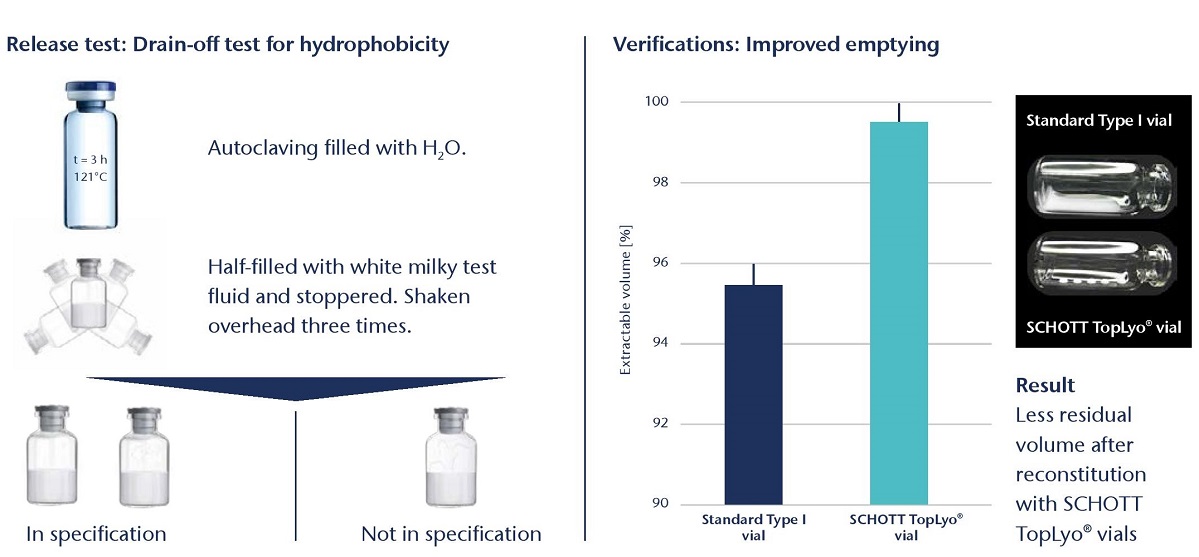
Cost reduction due to reduced overfilling - with lower residual volume
-
Reduced glass breakage due to refined geometry
-
Elimination of lyo-cake disruption and sidewall fitting for improved automated inspection
-
Lyocake stability during transport
-
Reduction of protein aggregation compared to siliconized glass vials
Learn more?
Would you like to learn more about SCHOTT AG products or do you have any questions? Do you need an individual offer or information about ordering, delivery and payment conditions? Contact us by phone, email or live chat.