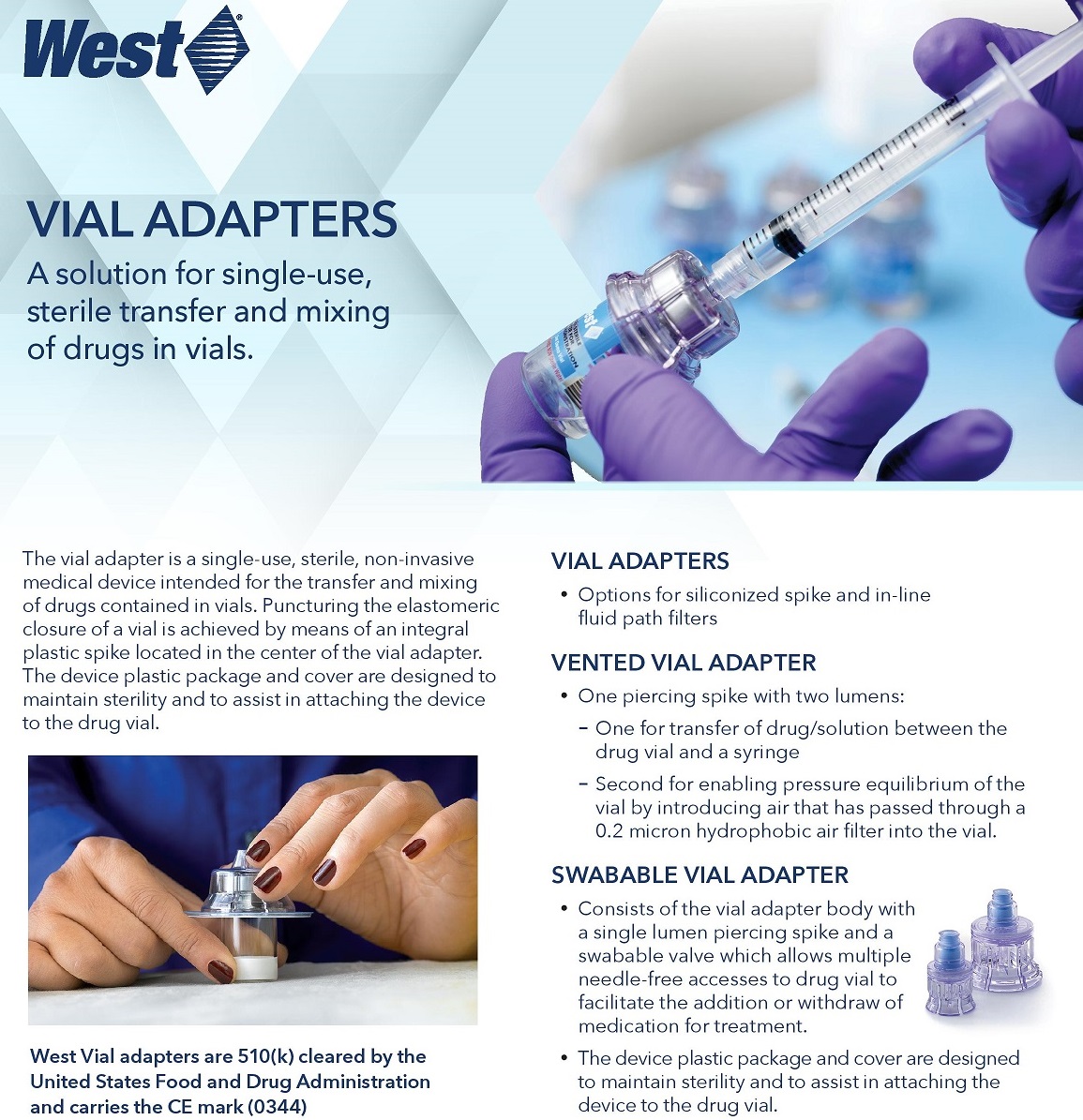
A solution for single-use, sterile transfer and mixing of drugs in vials.

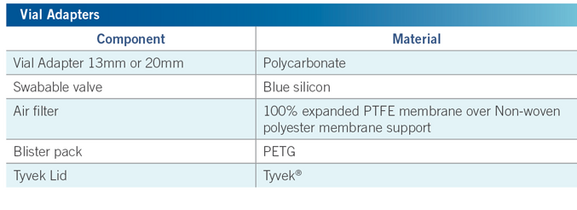
The Vial Adapter™ is a single-use, sterile, non-invasive medical device intended for the transfer and mixing of drugs contained in vials.
Puncturing the elastomeric closure of a vial is achieved by means of an integral plastic spike located in the center of the vial adapter. The device plastic package and cover are designed to maintain sterility and to assist in attaching the device to the drug vial.
Vial Adapter™ are available for all injection vials with a neck diameter of 13 mm and 20 mm.
-
Reduces the risk of needlestick injuries
-
Requires fewer parts and sharps
-
Options for siliconized spikes to reduce insertion force and inline fluid filters to reduce particle transfer
Vial Adapters™ are 510(k) cleared by the United States Food and Drug Administration and carry the CE mark (0344). The products are displayed for informational purposes only and may not be approved for marketing in certain regions. Distribution and use are subject to applicable regulatory approvals and requirements for medical devices. The Vial Adapter™ is configurable and may not be suitable for use with all medications. Refer to the drug manufacturer's labeling and instructions for use for device configuration compatibility. Failure to follow the product instructions for use may result in compromised sterility, contamination, leakage (including possible drug exposure), and inadequate drug reconstitution, transfer, and/or dosage. Product misuse could potentially result in user and/or patient infection, suboptimal therapy, and/or delay in therapy.
-
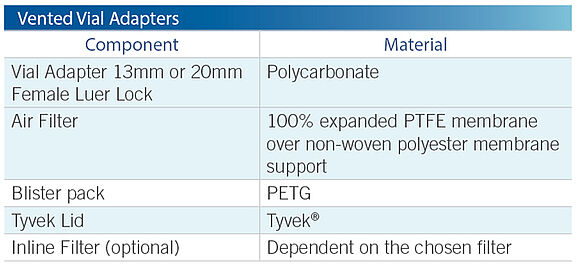
Dual-channel spike enables fast, high-volume withdrawal without pressure on the vial
-
Reduces splash back potential by equalizing pressure
-
PTFE 0.2 micron hydrophobic air filter
Vented Vial Adapters™ are 510(k) cleared by the U.S. Food and Drug Administration and bear the CE Mark (0344). The products are displayed for informational purposes only and may not be approved for marketing in certain regions. Distribution and use are subject to applicable regulatory approvals and requirements for medical devices. The Vented Vial Adapter™ is configurable and may not be suitable for use with all medications. Refer to the drug manufacturer's labeling and instructions for use for device configuration compatibility. Failure to follow the product instructions for use may result in compromised sterility, contamination, leakage (including possible drug exposure), and inadequate drug reconstitution, transfer, and/or dosage. Product misuse could potentially result in user and/or patient infection, suboptimal therapy, and/or delay in therapy.
-
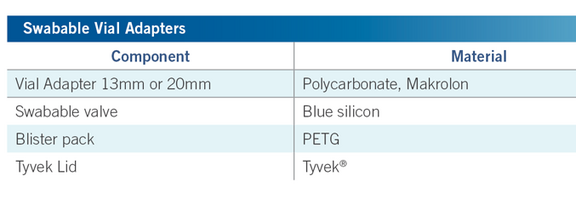
Contains a silicone rubber valve with Luer compatible connection.
-
Valve opens only when connected / compressed with standard luer slip or luer lock syringes
Swabable Vial Adapters™ are 510(k) cleared by the U.S. Food and Drug Administration and bear the CE Mark (0344). The products are displayed for informational purposes only and may not be approved for marketing in certain regions. Distribution and use are subject to applicable regulatory approvals and requirements for medical devices. The Swabable Vial Adapter™ prevents entry of microbes for up to 24 hours when used according to the product instructions for use and proper aseptic technique. Use of the Swabable Vial Adapter™ should not be interpreted as a modification, extension, or replacement of a drug manufacturer's labeling recommendations for storage and expiration. The Swabable Vial Adapter™ is not compatible for use with all medications. Refer to the drug manufacturer's labeling and instructions for use for information regarding shelf life and sterility of the opened or reconstituted drug product, as well as device compatibility. Failure to follow the product instructions for use may result in compromised sterility, contamination, leakage (including possible drug exposure), and inadequate drug reconstitution, transfer, and/or dosage. Product misuse could potentially result in user and/or patient infection, suboptimal therapy, and/or delay in therapy.
Learn more?
Would you like to learn more about West Pharmaceutical Services products or do you have any questions? Do you need an individual offer or information about ordering, delivery and payment conditions? Contact us by phone, email or live chat.